Resources
About Us
Europe Pharmaceutical Processing & Packaging Equipment Market by Mode of Delivery (Oral, Parenteral, Topical), Secondary Packaging (Cartoning, Labelling, Serialization, Wrapping) and End-of-Line Packaging (Palletizing, Case Packaging) - Forecast to 2032
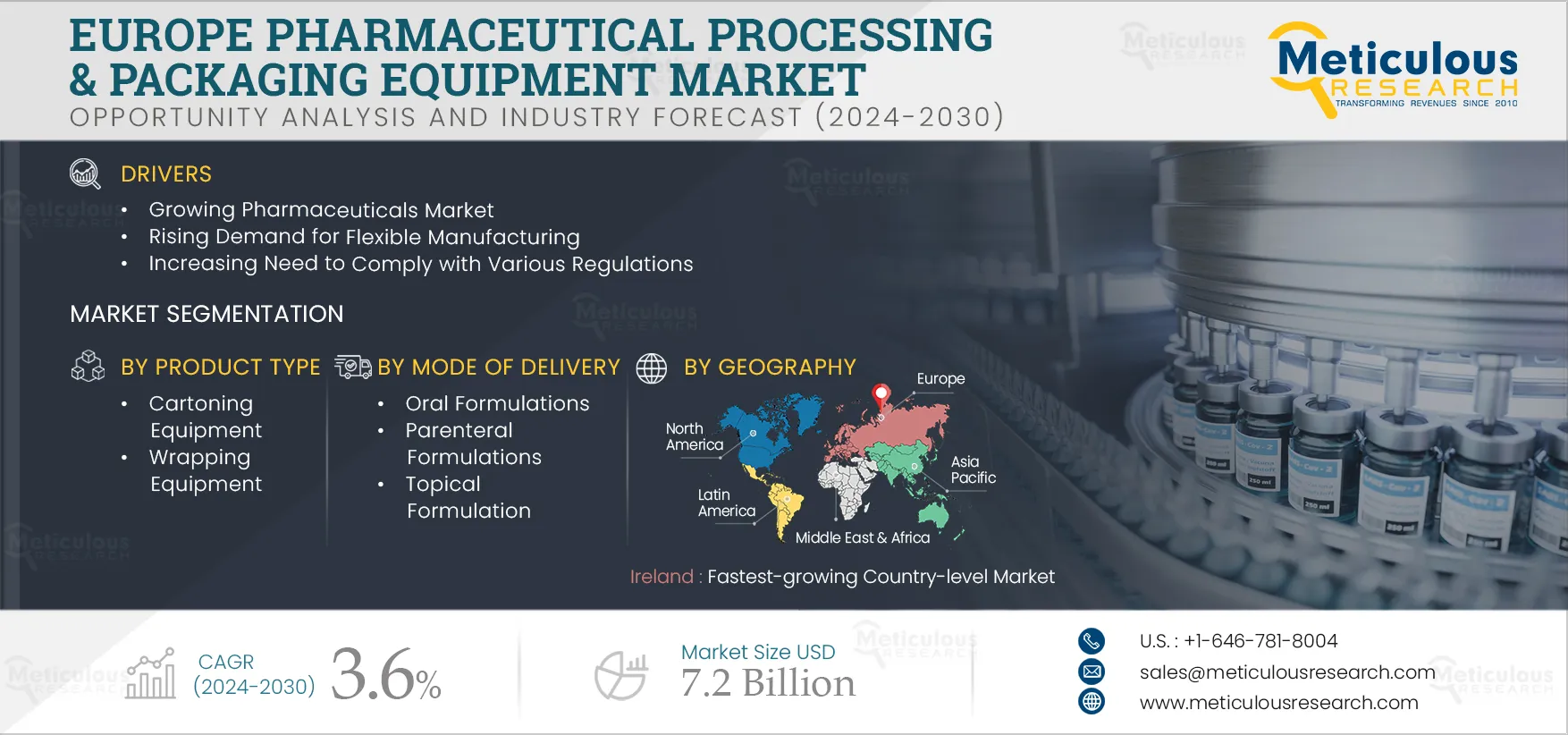
Report ID: MRHC - 104865 Pages: 250 Jan-2025 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe Europe Pharmaceutical Processing and Packaging Equipment Market is projected to reach $7.2 billion by 2032, at a CAGR of 3.6% from 2025 to 2032. Pharmaceutical processing and packaging equipment plays a crucial role in ensuring the quality and safety of pharmaceutical products. These machines are designed to meet strict industry standards and regulations, ensuring that the drugs produced are safe, effective, and high-quality. Packaging equipment provides protective barriers that safeguard the integrity of drugs during storage, transportation, and distribution.
The growth of this market is driven by the growing pharmaceuticals market, rising demand for flexible manufacturing, increasing need to comply with various regulations, and growing trend of contract manufacturing in the pharmaceutical sector. However, the preference for refurbished equipment restrains this market's growth to a significant extent. Additionally, emerging pharmaceutical manufacturing hotspots and growing pharmaceutical R&D spending offer opportunities for market growth. However, changing market demands in the constantly evolving pharmaceutical space is the major challenge for market growth.
Recent pharmaceutical trends support the development of specialized products for small groups of patients. Regulatory agencies support this trend by expediting drug approvals. Further, the increasing focus of pharmaceutical companies on cost control coupled with unpredictable demand for pharmaceuticals has created a need for more agile and flexible pharmaceutical manufacturing facilities. The emergence of continuous manufacturing and single-use technologies has supported the development of flexible manufacturing facilities, allowing for pharmaceutical processing and packaging innovation.
Fast-track drug approval programs and the need for operational flexibility among pharmaceutical companies and CMOs further support adopting flexible manufacturing processes. Orphan drug development is gaining traction due to its incentivization by regulatory authorities, accelerated drug approvals, and the exclusivity of molecules. Orphan drug developers focus on shortening the clinical development phase, wherein processes involving chemistry, manufacturing, control development, scale-up, validation, tech transfer, and stability testing must be completed rapidly after securing initial regulatory approval. Moreover, orphan drug development programs contribute to the increased adoption of orphan drugs. For instance, The European Medicines Agency (EMA) offers financial incentives for orphan drug development, including funding through the EU's research and innovation program, Horizon 2020. Such factors have increased the demand for flexible manufacturing in the pharmaceutical sector.
Thus, the increasing preference for specialized therapies among patients has boosted the adoption of flexible manufacturing practices involving single-use systems, continuous manufacturing systems, and modular facilities.
Click here to: Get Free Sample Pages of this Report
Growth in the aging population and the rapid increase in the burden of chronic and infectious diseases have increased the demand for pharmaceuticals. According to Eurostat, the population of Europe in 2021 was 747.8 million, out of which around 22.8% share was contributed by elderly people (above 65), which is expected to increase to 40.6% by 2050. In 2022, the geriatric population (aged 65 or over) accounted for a share of 21.1% of the total population in Europe, which increased from 20.8% in 2021. In 2022, Italy (23.8%), Portugal (23.7%), Finland (23.1%), Greece (22.7%), and Croatia (22.5%) had the highest share of the geriatric population. Consistent growth in the aging population is increasing the burden of chronic diseases. Common conditions affecting the geriatric population include osteoarthritis, diabetes, heart diseases, chronic obstructive pulmonary disorders, depression, and dementia. The rising prevalence of these conditions is increasing the demand for pharmaceuticals, thereby driving the pharmaceutical processing and packaging equipment market.
Chronic diseases are a leading cause of disability, illness, and death globally, with cardiovascular diseases, cancer, diabetes, and chronic obstructive pulmonary diseases (COPD) being the most common chronic disorders. Cancer is the leading cause of death globally. According to the GLOBOCAN, in Europe, the new cancer cases will reach 3,830,683 cases in 2032 from 3,266,461 cases in 2020, for the age group of 60 to 85+ years of age. Thus, the growing burden of chronic diseases has increased the consumption of pharmaceuticals, positively impacting the demand for pharmaceutical processing and packaging equipment.
Key Findings of the Europe Pharmaceutical Processing and Packaging Equipment Market Study:
In 2025, the Oral Formulations Segment is Expected to Dominate the Europe Pharmaceutical Processing and Packaging Equipment Market
Among the mode of drug delivery, in 2025, the oral formulations segment is expected to account for the largest share of the Europe pharmaceutical processing and packaging equipment market. Oral formulations include oral solid dosages and oral liquid dosages. Factors contributing to the segment’s largest share include patients' high preference for oral formulations due to their advantages, including ease of administration, high patient compliance, non-painful and better safety, lowest variability, and accurate dosing.
In 2025, the Cartoning Equipment Segment is Expected to Dominate the Europe Secondary Packaging Equipment Market
Among the types of secondary packaging equipment, in 2025, the cartoning equipment segment is expected to account for the largest share of the Europe pharmaceutical processing and packaging equipment market. Cartoning equipment is used to pack the primarily packaged pharmaceuticals to provide extra protection. It helps protect the products during shipment in a very cost-effective & efficient way. Regulatory agencies, such as U.S. FDA, also recommend using secondary packaging of pharmaceuticals, such as cartons, to protect the medicines from moisture, light, or any possible contaminants. Hence, cartons are considered an ideal secondary packaging for product handling, increasing their utilization and contributing to the segment’s largest share.
In 2025, the Case Packaging Equipment Segment is Expected to Dominate the Europe End-of-Line Packaging Equipment Market
Among the types of end-of-line packaging equipment, in 2025, the case packaging equipment segment is expected to account for the largest share of the Europe pharmaceutical processing and packaging equipment market. Factors such as the importance of case packaging in bulk packaging, supply chain efficiency, automation benefits, versatility, and the overall growth of the pharmaceutical industry contribute to the segment’s growth. Moreover, regulatory compliance also contributed to the segment’s growth as according to European Medicines Agency (EMA), there are guidelines and regulations related to Good Manufacturing Practice (GMP) for the manufacturing of pharmaceutical products, including requirements for processing and packaging equipment which are needed to be followed by the companies.
Ireland: Fastest-growing Country-level Market
Based on geography, Ireland is slated to record the highest growth rate during the forecast period. The growth of this market is mainly driven by the growing population in the country and the need for drugs by the growing population due to the prevalence of chronic disorders. For instance, the Ireland population reached 5.03 million in 2021 from 4,93 million in 2019. Moreover, the rising government activities to support research and innovation, availability of skilled professionals, increasing export of pharmaceuticals, and the rising pharmaceutical contract development and manufacturing partnerships in the country contribute to this region's market growth.
Key Players
The key players profiled in the sample preparation market report are Bausch+Ströbel (Germany), Korber AG (Germany), KORSCH AG (Germany), M.A.R. S.p.A. Macchine Automatiche Riempitrici (Italy), MAQUINARIA INDUSTRIAL DARA, SL (Spain), Marchesini Group S.p.A. (Italy), OPTIMA packaging group GmbH (Germany), Syntegon Technology GmbH (Germany), ANTARES VISION S.p.A. (Italy), BREVETTI CEA S.P.A. (Italy), CAM Packaging IT (Italy), Coesia S.p.A. (Italy), Fette Compacting (Germany), GEA GROUP (Germany), Glatt GmbH (Germany), LINXIS GROUP (France), Harro Höfliger Verpackungsmaschinen GmbH (Germany), I.M.A INDUSTRIA MACCHINE AUTOMATICHE S.P.A. (Italy), Uhlmann PacSysteme GmbH & Co. KG (Germany), and Tecnomaco Italia SRL (Italy).
Scope of the Report:
Europe Pharmaceutical Processing and Primary Packaging Equipment Market, by Mode of Delivery
Europe Pharmaceutical Secondary Packaging Equipment Market, by Product Type
Europe Pharmaceutical End-of-Line Packaging Equipment Market, by Product Type
Europe Pharmaceutical Processing and Packaging Equipment Market, by Country
Key questions answered in the report:
The Europe pharmaceutical processing & packaging equipment market study covers the market sizes & forecasts for various processing & packaging equipment used in pharmaceutical industries. The report includes the value analysis of various segments of the Europe pharmaceutical processing & packaging market at the country levels.
The Europe pharmaceutical processing & packaging equipment market is projected to reach $7.2 billion by 2032, at a CAGR of 3.6% during the forecast period.
Based on mode of drug delivery, the parenteral formulations segment is expected to grow at the highest CAGR due to their advantages over oral formulations, such as faster onset of action, precise drug delivery, increased drug stability, and greater absorption capacity.
Based on secondary packaging equipment, the wrapping equipment segment is expected to grow at the highest CAGR. Shrink-wrapping and over-wrapping are two major equipment used in the secondary pharmaceutical packing market, where both are important for protecting the product from external damage. Wrapping equipment also holds the cartons or bottles together, providing additional protection during handling, thus contributing to segment growth.
Based on end-of-line packaging equipment, the case packaging equipment segment is expected to grow at the highest CAGR. The growth in the pharmaceutical trade activities is a major factor contributing to the segment’s growth.
The growth of this market is driven by the advancements in sample preparation techniques, increasing adoption of process automation, development of bio-clusters to foster research, and increasing R&D investments among the end users. Additionally, emerging economies and personalized medicine offering growth potential are creating opportunities for market growth.
The key players operating in the sample preparation market are Bausch+Ströbel (Germany), Korber AG (Germany), KORSCH AG (Germany), M.A.R. S.p.A. Macchine Automatiche Riempitrici (Italy), MAQUINARIA INDUSTRIAL DARA, SL (Spain), Marchesini Group S.p.A. (Italy), OPTIMA packaging group GmbH (Germany), Syntegon Technology GmbH (Germany), ANTARES VISION S.p.A. (Italy), BREVETTI CEA S.P.A. (Italy), CAM Packaging IT (Italy), Coesia S.p.A. (Italy), Fette Compacting (Germany), GEA GROUP (Germany), Glatt GmbH (Germany), LINXIS GROUP (France), Harro Höfliger Verpackungsmaschinen GmbH (Germany), I.M.A INDUSTRIA MACCHINE AUTOMATICHE S.P.A. (Italy), Uhlmann PacSysteme GmbH & Co. KG (Germany), and Tecnomaco Italia SRL (Italy).
Countries such as Ireland and Denmark are projected to offer significant growth opportunities for vendors operating in this market due to increasing focus on pharmaceutical product development and manufacturing, growing adoption of technologically advanced products, and the rising export of products.
























Published Date: Apr-2024
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates