Resources
About Us
Alzheimer’s Drugs Market Size, Share, Forecast, & Trends Analysis by Drug (Cholinesterase Inhibitor [Donepezil, Galantamine, Rivastigmine], NMDA Receptor, Glutamate Regulator) Application (Disease Progression, Symptom Management) - Global Forecast to 2032
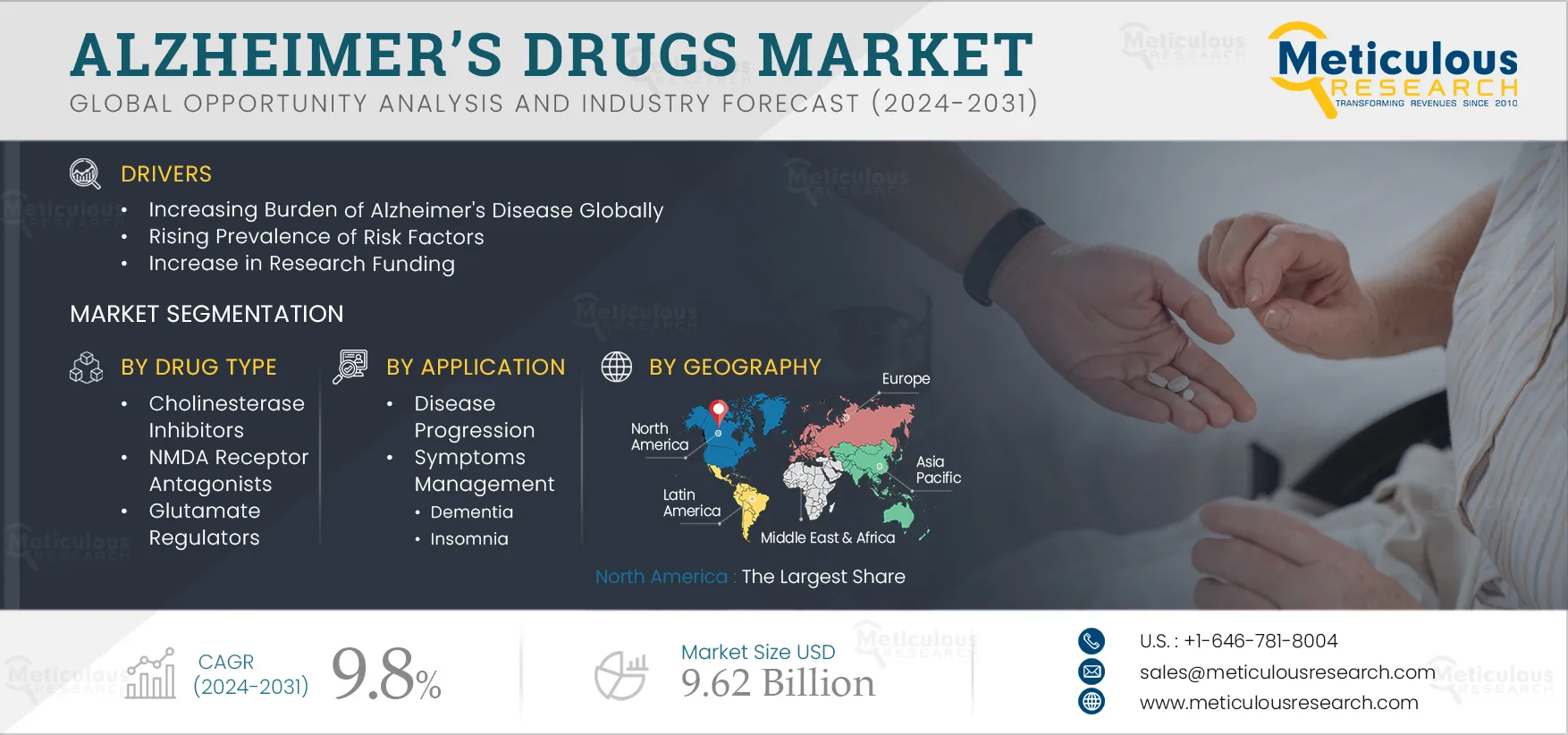
Report ID: MRHC - 1041264 Pages: 200 Sep-2024 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportThe Alzheimer’s Drugs Market is projected to reach $9.62 billion by 2032 at a CAGR of 9.8% from 2025 to 2032. The growth of this market can be attributed to various factors, such as the increasing burden of Alzheimer's disease globally, rising prevalence of risk factors, increase in research funding, introduction of innovative technologies, increasing number of approvals, and increasing number of clinical trials and pipeline products. Furthermore, growing research activities in developing countries, increase in awareness programs, initiatives by public and private organizations, use of combination drug therapy, and use of biomarkers and imaging techniques for diagnosis and management are expected to offer growth opportunities.
Alzheimer’s is a brain condition that causes a progressive decline in memory, organizing, learning, and thinking skills. It is the most common type of dementia. Neurodegenerative disorders are considered a major burden on the healthcare system and one of the major causes of disabilities across the globe. The prevalence of neurogenerative disorders is increasing owing to factors such as aging, traumatic brain injury, genetic factors, stress, and other lifestyle risk factors. According to Alzheimer's Association (U.S.), a non-profit organization focused on Alzheimer’s disease care, research, and support, in 2025, the prevalence of Alzheimer’s disease increased to 6.9 million in age 65 and older population from 6.2 million in 2021. Thus, the rising prevalence of Alzheimer’s disease is expected to increase the demand for Alzheimer’s drugs.
Increase in Research Funding
The increasing prevalence of Alzheimer’s disease leads to the rising need for novel therapies for its treatment. Many public and private players provide funding for Alzheimer’s disease research to understand the disease pathology and develop the drug accordingly. According to the Alzheimer’s Association (U.S.), Alzheimer's and dementia research funding at the National Institutes of Health (NIH) reached USD 3.8 billion in 2024 from USD 2.8 billion in 2021. Some of the recent funding are as follows:
 Click here to: Get Free Sample Pages of this Report
Click here to: Get Free Sample Pages of this Report
Regenerative medicine is proving to be an effective therapy for various medical conditions, including cardiovascular diseases, ophthalmic diseases, oncology, neurological diseases, immunology & inflammation, musculoskeletal diseases, dermatological diseases, and others. Stem cell therapy has emerged as a promising treatment for Alzheimer's disease. Stem cells are self-replicating and undifferentiated and can divide & develop, proving beneficial properties for Alzheimer’s drug development. Stem cell therapy has the potential to reduce the deposition of amyloid β-protein (Aβ), thereby alleviating symptoms of memory loss. In 2018, the first stem cell therapy was approved in Japan for the treatment of Alzheimer’s disease developed by South Korea-based companies Nature Cell and the Biostar Stem Cell Research Institute. Since then, the research on stem cell therapy for the treatment of Alzheimer’s disease has increased. Many of the stem cell therapies are in clinical trials for Alzheimer’s disease.
The traditional model of care for patients with Alzheimer’s disease involves long-term residential care, which can be more costly and not comfortable with many patients. Telehealth in Alzheimer’s disease involves the utilization of video conferencing to allow patients in remote locations to obtain treatment services. It provides patients and providers with a wide range of services, including consultations, clinical & educational programs, diagnosis and assessment, medication therapy management, and routine follow-up appointments. Patients who require convenient, affordable, and easily accessible mental health care can benefit from video-based telepsychiatry. Telehealth can manage symptoms of dementia at home before they turn into life-threatening issues. Many healthcare professionals use telehealth through video calls or mobile applications to remind patients about taking medications. Thus, increasing patient adherence to Alzheimer's disease drives the market growth of Alzheimer's drugs.
Combination therapy includes the combination of two approved and used therapeutic agents in a fixed combination dosage for the treatment of Alzheimer's disease. Recent advancements in understanding the genetics of Alzheimer’s disease revealed the number of pathways involved in increasing the risk of Alzheimer’s disease. Targeting these pathways together in combination has the potential to provide an advantage for Alzheimer’s disease patients. At present, combined therapy with memantine plus one cholinesterase inhibitor (ChEI) is considered an effective treatment for low to moderate Alzheimer’s disease. In many randomized-controlled clinical trials (RCTs), this combination has demonstrated higher clinical efficacy than monotherapy.
Based on drug type, the Alzheimer’s drugs market is segmented into cholinesterase inhibitors, NMDA receptor antagonists, glutamate regulators, and combination drugs. In 2025, the cholinesterase inhibitors segment is expected to account for the largest share of 46.9% of the Alzheimer’s drugs market. The drug works by decreasing the breakdown of acetylcholine, thus helping in the improvement of cognitive functions. The rising prevalence of Alzheimer’s disease and the increasing aging population are contributing to the demand for Cholinesterase inhibitors. The large share of the segment can be attributed to the high usage of these drugs for Alzheimer's treatment and the high number of drugs approved under this segment. At present, galantamine, donepezil, rivastigmine, and others are currently approved for the treatment of mild-to-moderate Alzheimer's disease (AD). Several studies have proven their efficacy for the treatment of the disease. According to an article published in the National Library of Medicine in 2022, cholinesterase inhibitors can slightly delay the loss of brain function in people who have mild to moderate Alzheimer’s disease.
Based on application, the Alzheimer’s drugs market is segmented into disease progression and symptom management. In 2025, the symptoms management segment is expected to account for the largest share of 87.2% of the Alzheimer’s drugs market. Symptom management is considered an important step in Alzheimer’s disease treatment. The majority of the drugs used in the treatment of Alzheimer’s disease are for symptom management, thus contributing to the largest share of the segment. The symptom management drugs slow down the process of disease progression, thus helping in reducing the healthcare cost associated with it, thus increasing demand. Alzheimer's disease symptoms such as impaired judgment, memory loss, language loss, and other brain changes can make it harder to manage other health conditions, which can create further complications. Hence, the demand for symptom management is expected to increase.
North America to Dominate the Market
In 2025, North America is expected to account for the largest share of the 43.0% of Alzheimer’s drugs market. The large share of the region is attributed to the increasing prevalence of Alzheimer’s disease, increasing funding for the development of Alzheimer’s treatment, the presence of key players, the rising number of clinical trials for Alzheimer’s drugs, and favorable reimbursement policies. For instance, in February 2025, the Centers for Medicare & Medicaid Services (U.S.), a federal agency within the United States Department of Health and Human Services that administers the Medicare program, covered a recently approved medication Leqembi (Lecanemab) used in the treatment of Alzheimer’s diseases. The drug is developed in partnership with Eisai Co., Ltd. (Japan) and Biogen Inc. (U.S.).
Moreover, the market in Asia-Pacific is slated to register the highest growth rate of 11.4% during the forecast period. The countries in the region, including China, India, and South Korea, are expected to offer significant opportunities for market growth. The growth in the country market is attributed to the growing pharmaceutical industry, supporting government initiatives, and a large number of clinical trials conducted in the region for Alzheimer’s. Also, government bodies in the region are providing funds for research on Alzheimer’s disease, which is driving the market’s growth. For instance, in August 2024, Bredis Healthcare (South Korea) received funding of USD 1.27 million for the development of diagnostic tests for Alzheimer's disease. The funding is provided by the South Korean Ministry of SMEs and Startups as a part of the Deep Tech Incubator Program for Startups (TIPS). Similarly, in May 2022, the Indian Health Service provided funding of USD 5 million to support urban Indian organizations, tribes, and tribal organizations to develop sustainable and comprehensive approaches to addressing Alzheimer’s disease.
The report offers a competitive landscape based on an extensive assessment of the product offerings and geographic presence of leading market players and the key growth strategies adopted by them over the past few years (2020–2025). The key players operating in the Alzheimer’s drugs market are Biogen (U.S.), Novartis AG (Switzerland), F. Hoffmann-La Roche AG (Switzerland), AbbVie Inc. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), Eisai Co., Ltd. (Japan), SUPERNUS PHARMACEUTICALS, INC. (U.S.), H. Lundbeck A/S (Denmark), AC Immune SA (Switzerland), Zydus Lifesciences Limited (India), Johnson & Johnson (U.S.), and Daiichi Sankyo Company (Japan).
Alzheimer’s Drugs Market Overview: Latest Developments from Key Industry Players
In July 2024, the U.S. FDA approved lecanemab through the Accelerated Approval Pathway for the treatment of Alzheimer’s disease. Lecanemab removes a sticky protein from the brain that is believed to cause Alzheimer’s disease progression. Eisai Co., Ltd. (Japan) and Biogen Inc. (U.S.) developed the drug in partnership.
|
Particulars |
Details |
|
Number of Pages |
200 |
|
Format |
|
|
Forecast Period |
2025-2032 |
|
Base Year |
2024 |
|
CAGR |
9.8% |
|
Estimated Market Size (Value) |
$9.62 billion by 2032 |
|
Segments Covered |
By Drug Type
By Application
|
|
Countries Covered |
North America (U.S. and Canada), Europe (Germany, France, U.K., Italy, Spain, Switzerland, Belgium, and Rest of Europe), Asia-Pacific (China, Japan, India, South Korea, and Rest of Asia-Pacific), Latin America (Brazil, Mexico, Argentina, Rest of Latin America), and Middle East & Africa (UAE, Saudi Arabia, South Africa, and Rest of Middle East & Africa). |
|
Key Companies |
The key players operating in the Alzheimer’s drugs market are Biogen (U.S.), Novartis AG (Switzerland), F. Hoffmann-La Roche AG (Switzerland), AbbVie Inc. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), Eisai Co., Ltd. (Japan), SUPERNUS PHARMACEUTICALS, INC. (U.S.), H. Lundbeck A/S (Denmark), AC Immune SA (Switzerland), Zydus Lifesciences Limited (India), Johnson & Johnson (U.S.), and Daiichi Sankyo Company (Japan). |
Key questions answered in the alzheimer’s drugs market report:
The Alzheimer’s drugs market report covers the qualitative and quantitative analysis of the market. It involves analyzing various market segments of Alzheimer’s drugs, such as type, source, production process, and end user, at the regional and country levels. The report also provides insights on factors impacting market growth, regulatory analysis, pipeline analysis, and Porter’s five forces analysis.
The Alzheimer’s drugs market is projected to reach $9.62 billion by 2032 at a CAGR of 9.8% from 2025 to 2032.
Among all the drug types studied in this report, in 2025, the cholinesterase inhibitors segment is expected to account for the largest share of 46.9% of the Alzheimer’s drugs market. The largest share of the segment is attributed to the increasing aging population, rising risk factors for Alzheimer's disease, increasing effectiveness of cholinesterase inhibitors, rising availability of cholinesterase inhibitors drugs, and rising accessibility and availability of cholinesterase inhibitors.
The growth of this market can be attributed to various factors, such as the increasing burden of Alzheimer's disease globally, rising prevalence of risk factors, increase in research funding, introduction of innovative technologies, increasing number of approvals, and increasing number of clinical trials and pipeline products. Furthermore, growing research activities in developing countries, increase in awareness programs, initiatives by public and private organizations, use of combination drug therapy, and use of biomarkers and imaging techniques for diagnosis and management are expected to offer growth opportunities.
The key players operating in the Alzheimer’s drugs market are Biogen (U.S.), Novartis AG (Switzerland), F. Hoffmann-La Roche AG (Switzerland), AbbVie Inc. (U.S.), Teva Pharmaceutical Industries Ltd. (Israel), Eisai Co., Ltd. (Japan), SUPERNUS PHARMACEUTICALS, INC. (U.S.), H. Lundbeck A/S (Denmark), AC Immune SA (Switzerland), Zydus Lifesciences Limited (India), Johnson & Johnson (U.S.), and Daiichi Sankyo Company (Japan).
Among all the geographies studied in this report, Asia-Pacific is slated to register the highest growth rate of 11.4% during the forecast period. The growth in the region is attributed to the increasing incidence of Alzheimer’s disease, growing research activities, increased awareness, a large population, rising healthcare expenditures, technological advancements, and government support for clinical trials.
























Published Date: Jan-2025
Published Date: Nov-2013
Published Date: Jul-2018
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates