Resources
About Us
Sterilization Equipment Market Size, Share, Forecast, & Trends Analysis by Offering (Equipment [Heat {Moist (Gravity, High-speed), Dry (Static Air, Forced Air)}, Low-temperature {ETO, Formaldehyde}, Radiation, Filtration] Services – Global Forecast to 2035
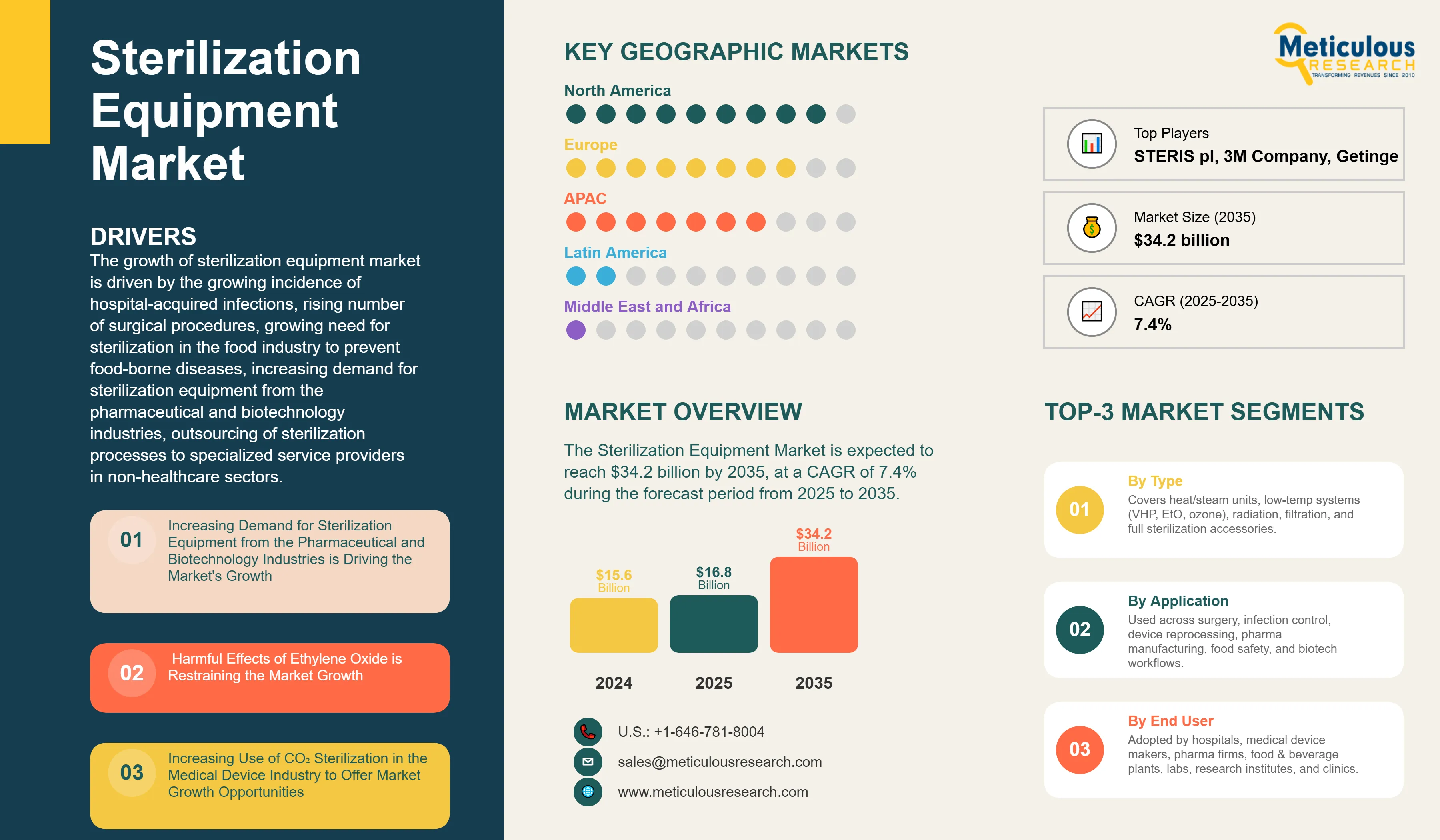
Report ID: MRHC - 104445 Pages: 334 Nov-2025 Formats*: PDF Category: Healthcare Delivery: 2 to 4 Hours Download Free Sample ReportThe Sterilization Equipment Market was valued at $15.6 billion in 2024. This market is expected to reach $34.2 billion by 2035 from an estimated $16.8 billion in 2025, at a CAGR of 7.4% during the forecast period from 2025 to 2035.
The growth of sterilization equipment market is driven by the growing incidence of hospital-acquired infections, rising number of surgical procedures, growing need for sterilization in the food industry to prevent food-borne diseases, increasing demand for sterilization equipment from the pharmaceutical and biotechnology industries, outsourcing of sterilization processes to specialized service providers in non-healthcare sectors. However, the market growth is restrained by harmful effects of ethylene oxide and concerns regarding the safety of reprocessed instruments. Moreover, the rising adoption of e-beam sterilization, growing demand for sterilization products in emerging markets, rising utilization of CO2 sterilization technology in the medical device industry, and increased focus on infection control in both healthcare and non-healthcare sectors are expected to generate growth opportunities for market stakeholders. Non-compliance with sterilization standards in the healthcare sector and the shift from reusable medical devices to disposable medical devices pose challenges to the growth of the market.
Click here to: Get Free Sample Pages of this Report
Increasing Demand for Sterilization Equipment from the Pharmaceutical and Biotechnology Industries
Companies in the pharmaceutical and biotechnology industries are key end users of sterilization technologies, as these solutions are essential for maintaining sterile processing environments in pharmaceutical manufacturing facilities. As the pharmaceutical and biotechnology sectors grow, the products being developed, including biologics, gene therapies, and advanced therapeutics, have become increasingly complex.
Contamination risks present significant challenges for both manufacturers and patients, resulting in the increased demand for effective sterilization solutions. The growing focus on higher quality standards and the increasing prevalence of infections linked to compromised manufacturing environments are driving the demand for advanced sterilization techniques and related equipment in the development and manufacturing processes of pharmaceutical and biopharmaceutical products.
Furthermore, the demand for biologics is increasing due to their effectiveness in treating various diseases. However, the need for the sterile manufacturing of these sensitive molecules presents significant challenges to manufacturers. Biologics must be produced under aseptic conditions, typically using sterile filtration, as they cannot withstand traditional sterilization methods such as heat, chemical treatments, or radiation. Additionally, unit operations during sterile production, such as pumping, can potentially damage and degrade biologics. Therefore, sterile manufacturing requires skilled professionals with extensive knowledge of sterilization equipment. As a result, many pharmaceutical companies are opting to outsource the sterilization of biologics to specialized providers.
Harmful Effects of Ethylene Oxide is Restraining the Market Growth
Sterilization of medical devices using ethylene oxide (EtO) is commonly preferred by healthcare organizations for instruments that cannot withstand heat sterilization or that contain electrical components sensitive to other sterilization methods. However, concerns about the safety of EtO as a sterilizing agent have grown, particularly following statements from the CDC indicating that repeated exposure to EtO may lead to skin and mucous membrane irritation, central nervous system abnormalities, and an increased risk of cancer in some cases.
Acute exposure to EtO is associated with symptoms such as headache, nausea, vomiting, cyanosis, and shortness of breath. These health concerns have led to a growing reluctance among healthcare organizations and other users to utilize the EtO sterilization method. In the U.S., the Environmental Protection Agency (EPA) has classified ethylene oxide as a potential human carcinogen and has restricted its use in specific sterilization applications.
Furthermore, the European Union has banned the use of ethylene oxide (EtO) in the food and beverage industry due to safety and environmental concerns. The demand for both EtO sterilization equipment and contract sterilization services is expected to decline in the coming years as regulatory authorities in various countries continue to tighten policies regarding the use of EtO for sterilization purposes.
EtO sterilization usage is declining, especially in the U.S., due to stricter environmental regulations. The U.S. EPA finalized emission control standards in April 2024 mandating 80% reduction in EtO emissions at sterilization plants. In the EU, REACH legislation classifies EtO as a “Substance of Very High Concern” (SVHC) under review for total phase-down by 2026.
These actions are pushing hospitals and contract sterilizers to transition toward vaporized hydrogen peroxide (VHP) and ozone technologies. Companies like Sterigenics and Sotera Health have invested in retrofitting EtO facilities with hybrid or alternative sterilization systems to maintain compliance.
Sterilization Equipment Market Trends
Use of Ozone Sterilization
Ozone is recognized as an oxidizing microbial agent with a strong bactericidal effect that can destroy microorganisms such as bacteria and viruses. Decontamination and disinfection using ozone significantly reduce the microbial load. Ozone is produced naturally when ultraviolet light or sunlight interacts with oxygen, as well as through electrical discharge. However, its use has historically been limited due to its instability. Recent technological advances have made ozone generation easier and more stable, leading to its increased application in sterilization.
Ozone sterilization is primarily used for medical devices, such as endoscopes, which cannot withstand the high heat and humidity associated with standard steam autoclaving. The advantages of ozone sterilization of ozone sterilization include the requirement of only medical -grade oxygen, which is safe to handle and transport and is readily available in hospitals worldwide, eliminating the need for costly sterilant stocking, shorter cycle time for ozone sterilization as compared to EtO sterilization, and lower costs per cycle as compared to EtO sterilization.
Sterilization Equipment Market Opportunity
Increasing Use of CO2 Sterilization in the Medical Device Industry to Offer Market Growth Opportunities
The demand for carbon dioxide (CO2) sterilization is expected to rise due to increasing concerns and restrictions regarding the use of ethylene oxide (EtO). CO2 is utilized in the form of supercritical carbon dioxide (scCO2), a state in which CO2 exhibits both gas and liquid-like properties. The supercritical state is achieved when pressure exceeds approximately 1,070 psi (73 atm) and temperature is above ~31°C.
ScCO2 sterilization effectively inactivates bacterial, viral, fungal, and yeast pathogens. The benefits of using scCO2 sterilization for medical devices include:
Furthermore, Supercritical CO2 (scCO2) sterilization remains one of the fastest-growing segments, advancing at an estimated CAGR of 9% between 2025 and 2032. The method’s low toxicity, absence of residue, and shorter cycle times make it attractive for implantable and biocompatible medical devices.
Since 2023, medical device manufacturers such as STERIS plc and 3M Company have launched scCO2-based prototype systems for shielding polymer-based materials and electronics-sensitive devices. The development of CO2-compatible packaging films by French and Japanese material technology firms is aiding adoption, particularly in reusable instrument processing.
Additionally, according to Qarboon, a France-based machine manufacturer, scCO2 is well-suited for the treatment of various medical devices, including textiles, silicone, plastics, bi-materials, and intelligent medical devices. ScCO2 has a strong impact on the biological load present on these devices and can be used to pre-sterilize both implantable and non-implantable, single-use/reusable medical devices. The growth of the medical device industry is expected to drive demand for scCO2 sterilization, creating opportunities for market growth.
Sterilization Equipment Market Study: Key Findings
The Equipment Segment to Account for the Largest Share of this Market in 2025
Based on offerings, the sterilization equipment market is segmented into equipment, services, and consumables & accessories. In 2025, the equipment segment is expected to account for the largest share of 55% of the sterilization equipment market. The substantial market share of this segment is due to the rising incidence of hospital-acquired infections, the high adoption of sterilization equipment in the medical devices and pharmaceutical industries, and the increasing need to ensure the safety of food & beverages.
The Pharmaceutical and Biotechnology Companies Segment to Account for the Largest Share of this Market in 2025
Based on end users, the sterilization equipment market is segmented into hospitals & clinics, medical device companies, pharmaceutical companies, food & beverage industry, and other end users. In 2025, the hospitals & clinics segment is expected to account for the largest share of 36% of the sterilization equipment market. This segment's substantial market share is due to the high prevalence of HAIs, rising number of surgical procedures, growing demand for clean and quality healthcare facilities among people, and increasing burden of chronic diseases associated with an aging population. According to the World Health Organization (WHO), out of every 100 patients in acute care hospitals, seven patients in high-income countries and 15 patients in low- and middle-income countries will be affected by at least one healthcare-acquired infection (HAI) during their hospital stay.
Geographical Analysis
In 2025, North America to Dominate the Sterilization Equipment Market
Based on geography, the global sterilization equipment market is segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. In 2025, North America is expected to account for the largest share of 45% of the global sterilization equipment market, followed by Europe and Asia-Pacific. The largest share of the region is attributed to the growing hospital & outpatient visits, rising number of surgical procedures, growing incidence of HAIs, increasing healthcare expenditure, growth in the pharma & biotech industries, and government initiatives to reduce HAIs and implement effective sterilization practices in the region.
Sterilization Equipment Market: Key Companies
The report includes a competitive landscape based on an extensive assessment of the key growth strategies adopted by leading market participants over the past three to four years. The key players profiled in sterilization equipment market report are STERIS plc (U.S.), 3M Company (U.S.), Getinge AB (Sweden), Cardinal Health, Inc. (U.S.), Advanced Sterilization Products, Inc. (U.S.) (A subsidiary of Fortive Corporation), Fedegari Autoclavi SpA (Italy), Stryker Corporation (U.S.), ANTONIO MATACHANA, S. A. (Spain), SteelcoBelimed AG (Switzerland), Sotera Health Company (U.S.), Benchmark Scientific Inc (U.S.), MMM Münchener Medizin Mechanik GmbH (Germany), Tuttnauer (Netherlands), and Consolidated Sterilizer Systems (U.S.), Andersen Sterilizers, Inc. (U.S.).
Sterilization Equipment Market Industry Overview: Latest Developments from Key Industry Players
|
Particulars |
Details |
|
Page No |
334 |
|
Format |
|
|
Forecast Period |
2025-2035 |
|
Base Year |
2024 |
|
CAGR |
7.4% |
|
Market Size (2025) |
$16.8 billion |
|
Market Size (2035) |
$34.2 billion |
|
Segments Covered |
By Offering
By End User
|
|
Countries Covered |
North America (U.S., Canada), Europe (Germany, France, U.K., Italy, Spain, Switzerland, Belgium, Rest of Europe), Asia-Pacific (China, Japan, India, Australia, South Korea, and Rest of Asia-Pacific), Latin America (Brazil, Mexico, and Rest of Latin America), and Middle East & Africa (Saudi Arabia, U.A.E., South Africa, and Rest of Middle East & Africa) |
|
Key Companies |
STERIS plc (U.S.), 3M Company (U.S.), Getinge AB (Sweden), Cardinal Health, Inc. (U.S.), Advanced Sterilization Products, Inc. (U.S.) (A subsidiary of Fortive Corporation), Fedegari Autoclavi SpA (Italy), Stryker Corporation (U.S.), ANTONIO MATACHANA, S. A. (Spain), SteelcoBelimed AG (Switzerland), Sotera Health Company (U.S.), Benchmark Scientific Inc (U.S.), MMM Münchener Medizin Mechanik GmbH (Germany), Tuttnauer (Netherlands), and Consolidated Sterilizer Systems (U.S.), Andersen Sterilizers, Inc. (U.S.) |
This study provides insights into sterilization equipment market segmented based on offering (equipment, services, consumables & accessories) offered by key companies to various end users, such as hospitals & clinics, medical device companies, pharmaceutical companies, food & beverage industry, and other end users. This report involves the value analysis of various segments and sub-segments of the market at the regional and country levels.
The sterilization equipment market is projected to reach $34.2 billion by 2035, at a CAGR of 7.4% during the forecast period.
The equipment segment is expected to account for the largest share of sterilization equipment market in 2025. Factors such as their effectiveness in sterilizing the reusable medical instruments, surgical instruments, and other materials required for various microbial-safe environments support this segment's largest share.
Based on end-user, the hospitals & clinics segment is projected to register the highest growth during the forecast period.
The growing incidence of hospital-acquired infections, rising number of surgical procedures, growing need for sterilization in the food industry to prevent food-borne diseases, increasing demand for sterilization equipment from the pharmaceutical and biotechnology industries, outsourcing of sterilization processes to specialized service providers in non-healthcare sectors are factors supporting the growth of this market. Further, the rising adoption of e-beam sterilization, growing demand for sterilization products in emerging markets, rising utilization of CO2 sterilization technology in the medical device industry, and increased focus on infection control in both healthcare and non-healthcare sectors are anticipated to offer significant growth opportunities for companies operating in this market.
The key players operating in sterilization equipment market are STERIS plc (U.S.), 3M Company (U.S.), Getinge AB (Sweden), Cardinal Health, Inc. (U.S.), Advanced Sterilization Products, Inc. (U.S.) (A subsidiary of Fortive Corporation), Fedegari Autoclavi SpA (Italy), Stryker Corporation (U.S.), ANTONIO MATACHANA, S. A. (Spain), SteelcoBelimed AG (Switzerland), Sotera Health Company (U.S.), Benchmark Scientific Inc (U.S.), MMM Münchener Medizin Mechanik GmbH (Germany), Tuttnauer (Netherlands), and Consolidated Sterilizer Systems (U.S.), Andersen Sterilizers, Inc. (U.S.).
























Published Date: Oct-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jun-2024
Published Date: Jun-2024
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates