Resources
About Us
Nepal IVD Market By Product (Kits, Instruments, Software), Technology (Immunoassay, Hematology, Coagulation, Diabetes, Molecular), Application (Infectious Diseases, Cardiology, Oncology), Customer Type (Hospital, Diagnostic Lab, Home Care) - Forecast to 2029
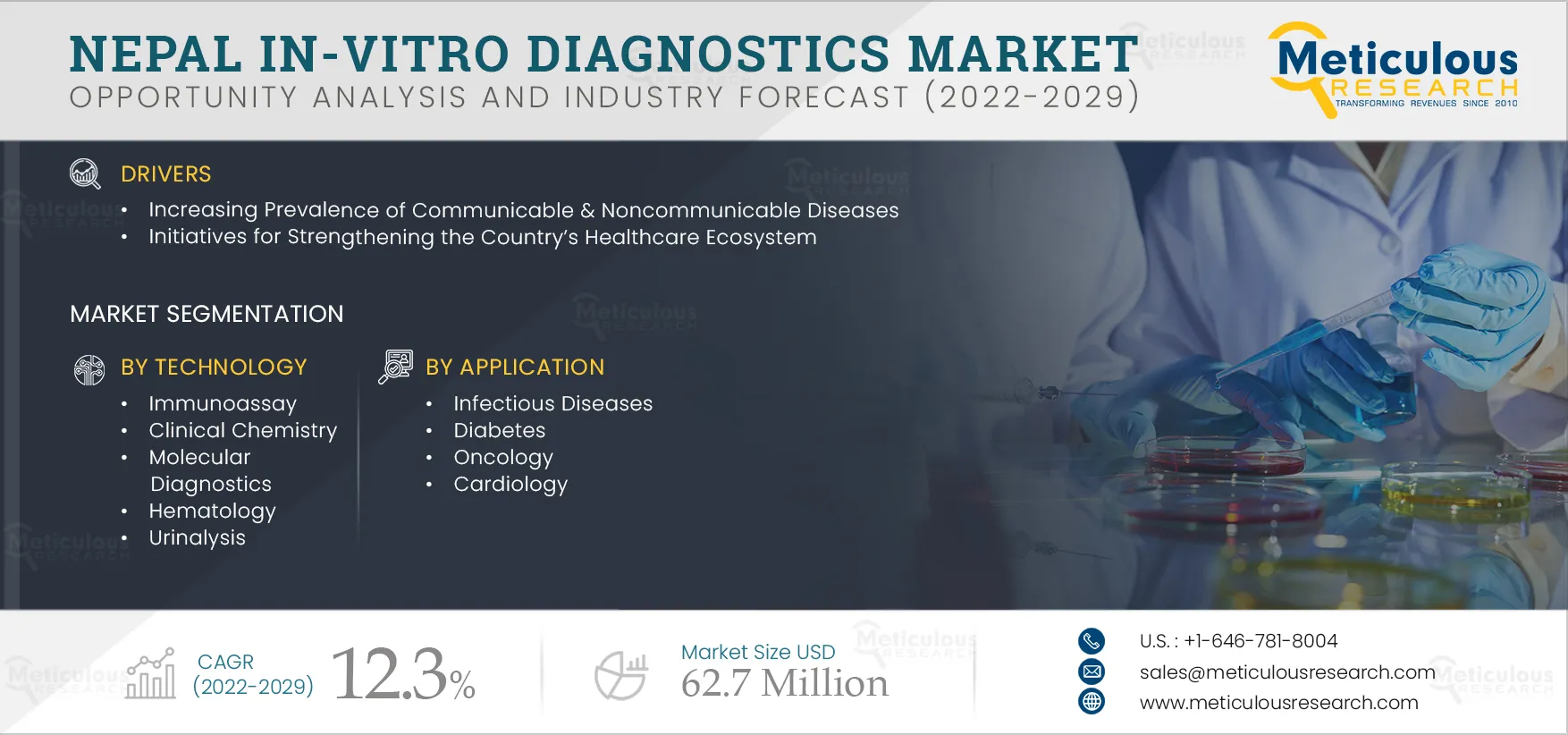
Report ID: MRHC - 104672 Pages: 122 Sep-2022 Formats*: PDF Category: Healthcare Delivery: 2 to 4 Hours Download Free Sample ReportThe Nepal IVD Market is expected to grow at a CAGR of 12.3% from 2022 to 2029 to reach $62.7 million by 2029. The growth of the Nepal in-vitro diagnostics market is mainly attributed to the increasing prevalence of infectious & non-communicable diseases and initiatives for strengthening the country’s healthcare ecosystem. However, the low accessibility & uneven distribution of healthcare services restrain the growth of this market. Additionally, the rising demand for quality healthcare in the country is expected to create market growth opportunities. However, the shortage of healthcare professionals is a major challenge for market growth.
When the first case of COVID-19 was identified in Nepal, there was not a single laboratory capable of performing RT-PCR tests. Samples were sent to other countries for testing, delaying the reports. The World Health Organization (WHO) helped the National Public Health Laboratory (NPHL) partner with a private research organization to provide RT-PCR primers and probes to NPHL. The NPHL started testing for COVID-19 using RT-PCR from 15th March 2020 onwards.
Further, with the additional joint efforts of the WHO and NPHL, 96 sites were established to facilitate RT-PCR testing. Moreover, the expansion of laboratory capacities also shows how the WHO’s country office worked together with the regional office (APAC), WHO Collaborating Centres associated with the WHO Global Influenza Surveillance and Response System (GISRS), the Global Outbreak and Response Network (GOARN), and Nepal’s Ministry of Health and Population to expand physical sites and ensure the quality of capacity expansion by enabling quality assurance mechanisms and establishing genome sequencing capabilities. The cases of COVID-19 infection decreased over time; therefore, these facilities performing RT-PCR tests and genome sequencing can be used to diagnose other infectious diseases such as tuberculosis and HIV-AIDS, which can help boost the demand for IVD products, driving the growth of Nepal’s IVD market.
Click here to: Get Free Sample Copy of this report
Increasing Prevalence of Communicable and Non-communicable Diseases Drives the Growth of Nepal In-vitro Diagnostics Market
The burden of non-communicable diseases (NCDs) is a major public health concern in Nepal. Behavioral risk factors such as alcohol consumption, smoking, and unhealthy diets are increasing the burden of non-communicable diseases in Nepal. Additionally, the prevalence of infectious diseases is also rising in the country. Several new infections, such as dengue fever, scrub typhus, H1N1 & H5N1 influenza, and HIV-TB coinfections, have emerged in the country in recent years, posing serious public health risks. The Government of Nepal has reported an increase in the cases of various infectious diseases.
Apart from infectious diseases, NCDs are a leading factor of morbidity and mortality in Nepal. Tobacco use, alcohol consumption, sedentary lifestyles, unhealthy diets, and social, environmental, and economic factors are increasing the prevalence of NCDs in the country. In 2019, the Government of Nepal, along with the World Health Organization, conducted a noncommunicable disease risk factor ‘STEP’ survey, which found high tobacco and alcohol consumption, a high percentage of people being overweight, and high blood pressure medications. Hence, the prevalence of NCDs, such as cancer, is increasing.
According to the Global Cancer Observatory, in 2020, cancer cases in Nepal reached 20,508. The number of cases is estimated to reach 32,029 by 2035. Also, according to the World Bank’s Global Health Estimates 2020, the share of total deaths in the country due to non-communicable diseases increased from 60% in 2015 to 66% in 2019. Thus, the increasing prevalence of infectious & noncommunicable diseases is expected to drive the demand for in-vitro diagnostic testing products for early disease diagnosis, prevention, and prognosis.
The molecular diagnostics segment is expected to propel with the fastest growth over the forecast period
The growth of the segment can be attributed to the technological advancements in molecular techniques and the establishment of PCR testing in the country due to the COVID-19 pandemic. Before the pandemic, no single PCR testing option was available in the country. The samples for PCR testing were sent to neighboring countries. The need for RT-PCR tests during the COVID-19 pandemic led the government to establish RT-PCR testing with support from WHO
The reagent, assays, and kits segment is projected to register the highest CAGR during the forecast period
The rising incidence of chronic and infectious diseases and the increasing portfolio of disease-specific kits are the key factors contributing to the largest share of reagents, assays, and kits.
The oncology segment is projected to register the fastest growth during the forecast period
The factors propelling the growth of the oncology segment include the increasing prevalence of cancer in the country. According to International Agency for Research on Cancer, in 2020, 20,508 new cancer cases were reported in Nepal, which is estimated to increase by 37.1% by the year 2030. Thus, the demand for IVD is expected to increase for oncology in Nepal.
The home care/self-testing segment is expected to grow at the highest CAGR during the forecast period
The rising availability of at-home test kits for several diseases, the growing geriatric population, rising awareness among patients regarding at-home testing, and the ongoing switch from hospital to home health care to curtail the rising costs are the factors responsible for the highest growth rate of this segment.
Key Players
The report includes a competitive landscape based on an extensive assessment of the product portfolio offerings, geographic presences, and key strategic developments in leading industry market players over the past years. The key players profiled in the Nepal in-vitro diagnostics market are F. Hoffman-La Roche Ltd (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Abbott Laboratories (U.S.), Danaher Corporation (U.S.), Becton, Dickinson and Company (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Agilent Technologies, Inc. (U.S.), PerkinElmer, Inc. (U.S.), Accurex Biomedical Pvt. Ltd (India), and Siemens Healthineers AG (Germany).
Scope of the Report:
Nepal In-vitro Diagnostics Market, by Product
Nepal In-vitro Diagnostics Market, by Technology
Nepal In-vitro Diagnostics Market, by Application
Nepal In-vitro Diagnostics Market, by Customer Type
Key questions answered in the report:
The Nepal in-vitro diagnostics market covers the market size & forecasts of reagents, assays, and kits, instruments/analyzers, software & services used for diagnostics in Nepal. Nepal in-vitro diagnostics market studied in this report involves the value analysis of various segments and sub-segments of the Nepal in-vitro diagnostics market.
Nepal in-vitro diagnostics market is projected to reach $62.7 million by 2029, at a CAGR of 12.3% during the forecast period.
Based on product, the reagents, assays, and kits segment is expected to account for the largest share of the Nepal in-vitro diagnostics market in 2022. Moreover, this segment is expected to grow at the highest CAGR during the forecast period.
Based on technology, the molecular diagnostics segment is projected to create more traction owing to the technological advancements in molecular diagnostics and government initiatives to establish molecular diagnostics in the country.
The increasing prevalence of infectious & noncommunicable diseases and initiatives to strengthen the country’s healthcare ecosystem are driving the market growth. In addition, the rising demand for quality healthcare in the country provides immense growth opportunities for players operating in the Nepal in-vitro diagnostics market.
The key players operating in the Nepal In-vitro Diagnostics Market are F. Hoffman-La Roche Ltd (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Abbott Laboratories (U.S.), Danaher Corporation (U.S.), Becton, Dickinson and Company (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Agilent Technologies, Inc. (U.S.), PerkinElmer, Inc. (U.S.), Accurex Biomedical Pvt. Ltd (India), and Siemens Healthineers AG (Germany).
























Published Date: Jun-2025
Published Date: Apr-2025
Published Date: Apr-2025
Published Date: Apr-2025
Published Date: Jan-2025
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates