Resources
About Us
Middle East IVD Market by Offering (Kits, Software), Technology (Immunoassay, Molecular Diagnostics [PCR, NGS, Microarray], Rapid Tests, Biochemistry), Application (Infectious Diseases, Oncology), Diagnostic Approach (Lab) - Forecast to 2032
Report ID: MRHC - 1041081 Pages: 200 Jan-2024 Formats*: PDF Category: Healthcare Delivery: 24 to 72 Hours Download Free Sample ReportIn vitro diagnostics (IVD) are tests performed on biological samples such as blood, saliva, and other tissues taken from the human body to detect a wide range of diseases. IVD tests are used to screen biological samples to monitor individuals’ health and prevent or diagnose diseases. These consist of various tests based on techniques such as enzyme-linked immunosorbent assay (ELISA), radioimmunoassay, polymerase chain reaction (PCR), isothermal nucleic acid amplification, and next-generation sequencing. These tests can be performed in laboratories, homes, and healthcare facilities.
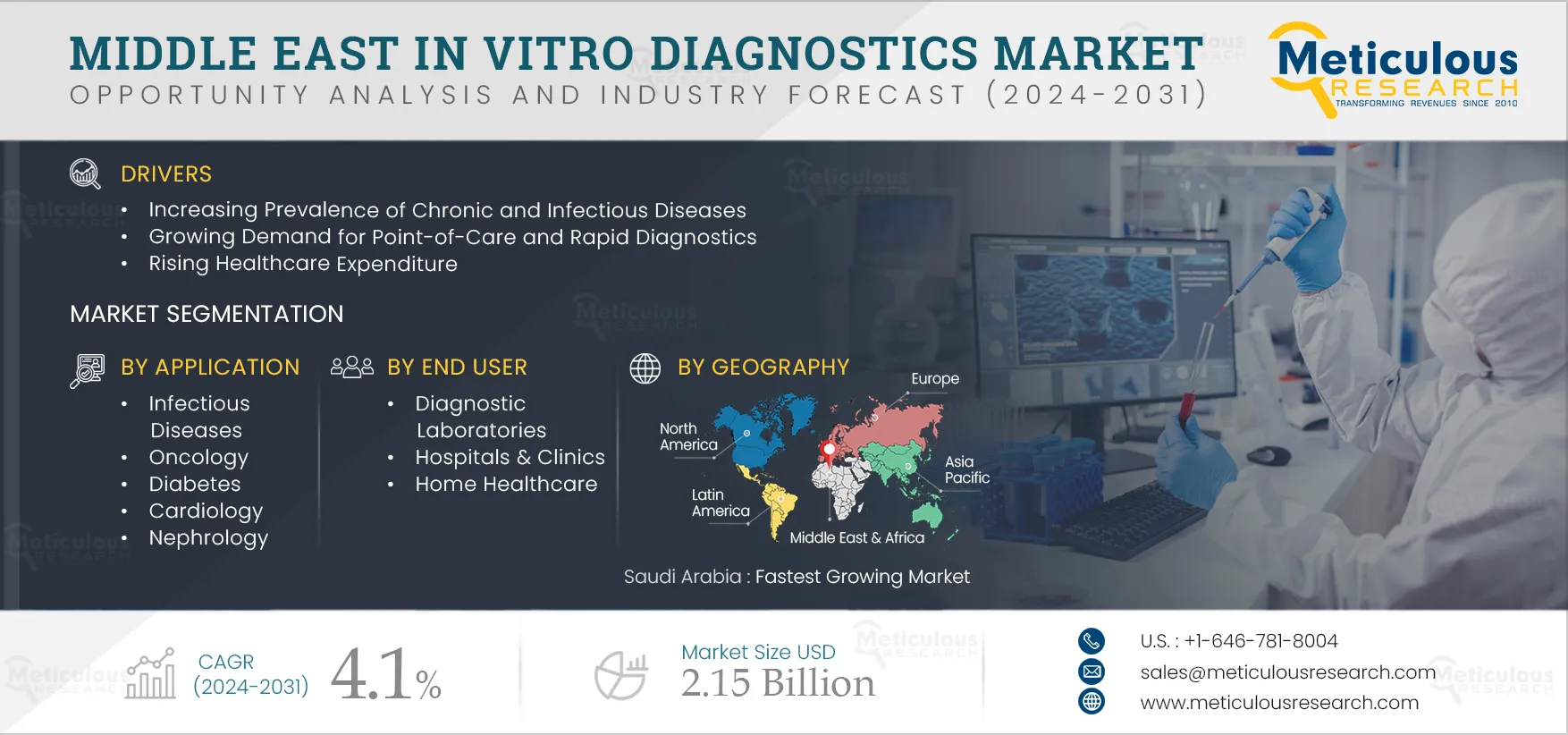
The growth of the Middle East IVD market is attributed to the rising prevalence of chronic and infectious diseases, the growing demand for point-of-care (PoC) and rapid diagnostics, rising healthcare expenditures, growth in the aging population, and rising awareness about healthcare. However, high prices for IVD tests and the variance in test results observed in rapid IVD tests restrain the growth of the IVD market.
The rising awareness regarding early disease diagnosis and increasing inclination toward personalized medicine in the UAE and Saudi Arabia are expected to create growth opportunities for the players operating in this market. However, the concerns pertaining to false positive results in immunoassays and POC are a major challenge for market growth.
Chronic diseases are often associated with the elderly population due to the declining bodily functions and immunity among this population segment. Aging is associated with progressive deterioration in the structure and functioning of organs. The elderly population is more prone to various chronic diseases. According to the World Bank, in 2022, in Iran, Saudi Arabia, and the UAE, the percentage of the elderly population out of the total population was 8%, 3%, and 2% respectively.
The prevalence of chronic diseases and conditions such as cancer, diabetes, arthritis, and heart disease are on the rise in Middle East countries. According to GLOBOCAN, in Israel, the prevalence of cancer is expected to increase to 44.2 thousand cases in 2040 from 28.7 thousand cases in 2020. Similarly, in Saudi Arabia, the prevalence of cancer is expected to increase to 60.4 thousand cases in 2040 from 27.9 thousand cases in 2020. Early detection is very important in reducing mortality in the case of cancer, improving the chances of survival, and saving on treatment costs. Various rapid diagnostic kits are used to detect cancer. The demand for IVD kits for cancer testing is expected to grow significantly in the coming years.
The growth in the geriatric population and social behaviors such as tobacco and alcohol consumption, physical inactivity, and unhealthy diets are major factors driving a steady increase in the number of people suffering from chronic diseases. The growth in the elderly population and the associated increase in the burden of chronic disease is increasing the demand for IVD testing in the Middle East.
Click here to: Get Free Sample Pages of this Report
Personalized/precision medicine uses an individual’s genomic information to offer targeted treatment. The synthesis of genes can rapidly sequence large sections of a person’s genome and aid in the formulation of precision medicine, in turn generating cost-effective alternatives for existing therapies and allowing for novel treatments in multiple areas of infectious diseases, such as sepsis, hepatitis, and cervical cancer. Personalized medicine analyzes molecules, individual genes, and networks and translates them into an overall output for the systems of the human body. In the Middle East region, the adoption of personalized medicine is increasing in the countries, such as Saudi Arabia and UAE is providing opportunity for market growth.
Personalized medicines benefit from infectious disease management practices guided by genomic information extracted from causing microbes. These medicines can tailor therapies with the best responses and highest safety margins, ensuring better patient care. Therapeutic drug monitoring tests to select drugs for resistant HIV strains are among the most widely used personalized treatment regimes.
The launch of personalized medicine programs by various government agencies in the region is driving market growth in the region. For instance, Saudi Arabia is working toward developing a genomics research infrastructure in line with its Saudi Vision 2032 initiative. The objectives of Vision 2032 are to capture the genetic blueprint in Saudi Arabia and revolutionize healthcare by enabling personalized medicine.
Based on offering, the Middle East IVD market is segmented into reagents and kits, instruments, and software & services. In 2025, the kits & reagents segment is expected to account for the largest share of the market. The large market share of this segment is attributed to the frequent use of reagents and kits in the detection of various chronic diseases, the commercial availability of a diverse range of reagents and consumables for the diagnosis of various diseases, ease of use of reagents & kits, and emerging threat of infectious diseases. For instance, according to WHO, between 2012 and October 2022, 2,600 laboratory-confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection have been identified, of which 84% (2,193/2,600) have been reported in Saudi Arabia. Thus, the emerging threat of infectious diseases is increasing the demand for kits & reagents for rapid testing.
Based on technology, the Middle East IVD market is segmented into immunoassay/immunochemistry, biochemistry/clinical chemistry, molecular diagnostics, point-of-care (POC) diagnostics, whole blood glucose monitoring, microbiology, hematology, coagulation/hemostasis, urinalysis, and other technologies. In 2025, the molecular diagnostics segment is expected to account for the largest share of the market. The growing prevalence of infectious diseases, technological innovations, and the advantages of molecular diagnostics tests, such as cost-effectiveness, rapid results, and high sensitivity and specificity over laboratory tests
Additionally, the strategic initiatives adopted by key players in the region are expected to support the largest share of the segment. In June 2021, G42 Healthcare (UAE) signed a Memorandum of Understanding (MoU) with Seegene Inc. (South Korea) to offer fully equipped mobile diagnostics and testing laboratories across the Middle East and North Africa (MENA) region. The partnership was aimed at providing the Seegene Mobile Station, a laboratory-on-wheels facility to provide optimized molecular diagnosis and tests across MENA, including the UAE, Algeria, Morocco, Tunisia, Libya, Egypt, Sudan, Palestine, Jordan, Syria, Iraq, Iran, Pakistan, Lebanon, Kuwait, Qatar, Oman, Saudi Arabia, Bahrain, and Yemen.
Based on application, the Middle East in vitro diagnostics (IVD) market is segmented into infectious diseases, oncology, diabetes, cardiology, nephrology, autoimmune disorders, and other applications. The cardiology segment is expected to register the highest CAGR during the forecast period. Cardiovascular diseases (CVD) remain the leading cause of death globally. IVD testing is used for biomarker detection in cardiology. The early-stage biomarkers of CVD can potentially save many lives and help alleviate the global burden of CVD. The increasing prevalence of cardiovascular diseases, the growing global geriatric population, and increasing research on cardiac biomarkers are expected to drive the growth of this segment.
Based on diagnostic approach, the Middle East IVD market is segmented into laboratory testing, OTC/self-testing, and point-of-care testing. In 2025, the laboratory testing segment is expected to account for the largest share of the market. The large market share of this segment is primarily attributed to its higher accuracy and reliability, lower costs, and availability of several IVD tests in laboratory settings. Additionally, the laboratory testing landscape is changing in Middle East countries; due to emerging threats of epidemics, governments, along with interest groups are working to increase the rate of laboratory testing in the region. For instance, in February 2024, GC Labs (South Korea) is planning to expand its network of diagnostic laboratories in the UAE and increase access to lab testing in the country.
Based on end user, the Middle East in vitro diagnostics (IVD) market is segmented into diagnostic laboratories, hospitals & clinics, home healthcare, and other end users, which include nursing homes, academic & research institutes, ambulatory care centers, and transfusion laboratories. The hospitals & clinics segment is expected to register the highest CAGR during the forecast period. The highest CAGR of this segment is attributed to the increasing number of hospitalizations due to various diseases requiring diagnosis and the rise in the number of hospitals and clinics in the region, leading to growth in the utilization of diagnostic products. For instance, as of 2022, in UAE, the number of private medical facilities reached 4,482, including 56 hospitals and 55,208 licensed medical professionals (Source: Dubai Healthcare City Authority).
Saudi Arabia is expected to register the highest CAGR during the forecast period. The highest CAGR of this regional market is attributed to the rising healthcare expenditure, the growing aging population, and the increasing adoption of self-testing. Additionally, the rising chronic and infectious diseases are increasing the demand for IVD. Chronic diseases such as ischemic heart disease, stroke, chronic obstructive pulmonary disease (COPD), and cancer are responsible for most deaths in Saudi Arabia. For example, in 2021, 4.3 million people aged 20–79 years were living with diabetes in Saudi Arabia. This number grew with a CAGR of 3.4% (from 2011–2021) from 2.8 million in 2011 (Source: IDF Diabetes Atlas 2021). Thus, the demand for IVD glucose testing is increasing in the country.
The report offers a competitive landscape based on an extensive assessment of the product portfolio offerings, geographic presences, and key strategic developments adopted by leading market players in the industry over the years. The key players operating in the Middle East IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), BioMérieux S.A. (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche (Switzerland), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Illumina, Inc. (U.S.), QuidelOrtho Corporation. (U.S.), Agilent Technologies, Inc. (U.S.), and DiaSorin S.p.A. (Italy).
|
Particular |
Details |
|
Number of Pages |
~200 |
|
Format |
|
|
Forecast Period |
2025-2032 |
|
Base Year |
2024 |
|
CAGR |
4.1% |
|
Estimated Market Size (Value) |
$2.15 billion by 2032 |
|
Segments Covered |
By Offering
By Technology
By Application
By Diagnostic Approach
By End User
|
|
Countries Covered |
Saudi Arabia, UAE, Qatar, Kuwait, Oman, Israel, and the Rest of the Middle East |
|
Key Companies |
The key players operating in the Middle East IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), BioMérieux S.A. (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche (Switzerland), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Illumina, Inc. (U.S.), QuidelOrtho Corporation. (U.S.), Agilent Technologies, Inc. (U.S.), and DiaSorin S.p.A. (Italy). |
The Middle East IVD market is segmented based on product & solution (reagents & kits, systems, and software & services) and a wide range of technologies (immunoassay/immunochemistry, biochemistry/clinical chemistry, molecular diagnostics, microbiology, hematology, coagulation/hemostasis, urinalysis, and other technologies) offered by key companies to various end users, such as diagnostic laboratories, hospitals & clinics, home healthcare, and other end users. The Middle East IVD market studied in this report involves the value analysis of various segments and sub-segments of in-vitro diagnostics at regional and country levels.
The Middle East IVD market is projected to reach $2.15 billion by 2032 at a CAGR of 4.1% from 2025 to 2032.
The kits & reagents segment is estimated to account for the largest share of the Middle East IVD market in 2025. Factors such as the high burden of diseases, the high adoption of rapid diagnostic test kits, and the presence of initiatives supporting early disease diagnosis are responsible for the largest market share.
Based on the technology, the molecular diagnostics segment is projected to create more traction owing to the technological innovations and the advantages of rapid immunoassay tests, such as cost-effectiveness, rapid results, and high sensitivity & specificity over laboratory tests.
Based on application, the cardiology segment is projected to create more traction during the forecast period due to the growing burden of cardiovascular diseases and rising demand for early diagnosis.
Based on end user, the hospitals & clinics segment is projected to create more traction during the forecast period due to a large number of hospitalizations due to various diseases, the rising geriatric population, increasing healthcare access & expenditure, and the rising prevalence of healthcare-associated infections (HAIs).
The growth of the Middle East IVD market is attributed to the rising prevalence of chronic and infectious diseases, the growing demand for point-of-care (PoC) and rapid diagnostics, rising awareness regarding early disease diagnosis, rising healthcare expenditures, and increasing funding for research activities. The rising awareness regarding early disease diagnosis and increasing inclination toward personalized medicine in the UAE and Saudi Arabia are expected to create growth opportunities for the players operating in this market.
The key players operating in the Middle East IVD market are Abbott Laboratories (U.S.), Becton, Dickinson and Company (U.S.), BioMérieux S.A. (France), Danaher Corporation (U.S.), F. Hoffmann-La Roche (Switzerland), QIAGEN N.V. (Netherlands), Siemens Healthineers AG (Germany), Thermo Fisher Scientific Inc. (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Illumina, Inc. (U.S.), QuidelOrtho Corporation. (U.S.), Agilent Technologies, Inc. (U.S.), and DiaSorin S.p.A. (Italy).
Saudi Arabia is expected to offer significant growth opportunities owing to the government initiatives to improve the healthcare infrastructure in the country, the rising prevalence of infectious diseases, and the growth in the geriatric population.
























Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Nov-2024
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates