Resources
About Us
Latin America IVD by Product & Solution, Technology (Immunoassay, Point of Care, Molecular Diagnostics), Application (Infectious Diseases, Diabetes, Oncology), Diagnostic Approach (Lab, OTC, PoC), End User - Forecast to 2030
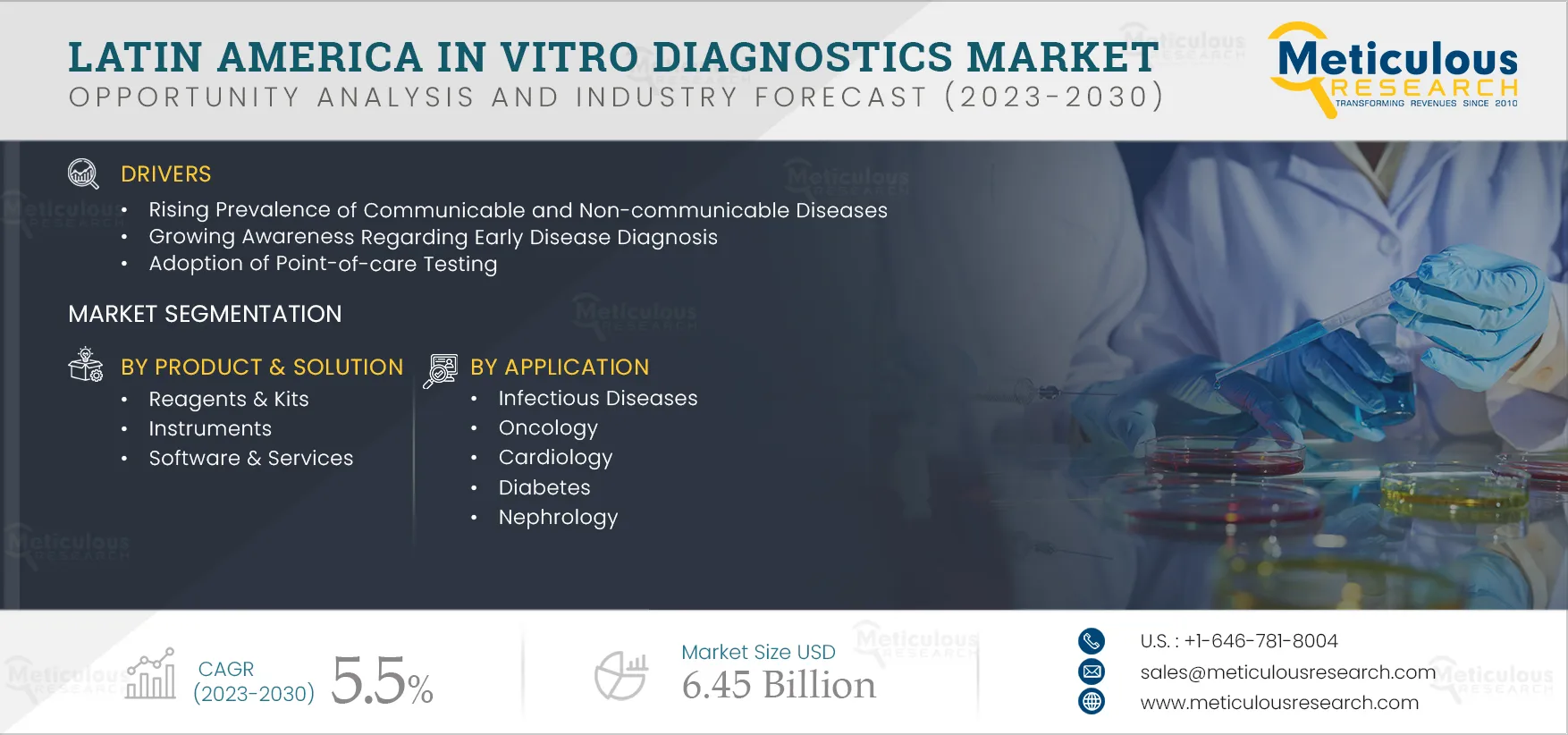
Report ID: MRHC - 104407 Pages: 205 Jul-2023 Formats*: PDF Category: Healthcare Delivery: 2 to 4 Hours Download Free Sample ReportThe Latin American IVD Market is expected to register a CAGR of 5.5% during the forecast period 2023–2030 to reach $6.45 billion by 2030. The burden of communicable and non-communicable diseases, the rise in medical tourism, the shift toward decentralized testing, and growing awareness regarding early disease diagnosis are driving the growth of this market.
In addition, the inclination toward personalized healthcare and advances in genomics and proteomics offer opportunities for the IVD market. However, the regulatory scenarios in LATAM countries are posing a challenge to the growth of this market.
Diagnosis is crucial for precision medicine to screen patients for breakthrough therapies and detect certain conditions, such as cancer, at an earlier stage. Personalized medicines for infectious diseases help orient the molecular management of infections.
The Personalized Health Index is a first-of-its-kind, data-driven policy tool that measures each country's progress toward the personalized future of healthcare. Uruguay held the first position in the Personalized Health Index for Latin America. Also, according to the Latin American Personalized Medicine Index, Mexico is one of the top five Latin American countries with the best conditions to integrate and promote personalized medicine.
Latin America performs best in personalized technologies such as devices, applications, and reimbursement structures that help drive personalized healthcare in the region. The countries in Latin America are progressing toward the adoption of personalized healthcare and medicine, providing opportunities for the IVD market in the region.
Click here to: Get a Free Sample Copy of this report
Growing Prevalence of Chronic and Infectious Diseases in the Region
Chronic infectious diseases caused by bacterial and viral pathogens are rising in Latin American countries due to various factors, including changes in climatic conditions, increased human population growth & density in urban & rural areas, increased human migration & mobility, and poor public health resources. The pathogenic agents that cause emerging and re-emerging diseases in Latin America include bacterial pathogens (Salmonella enteritidis, Salmonella typhimurium, Salmonella typhi, Brucellaabortus, and Klebsiella pneumonia) and viral pathogens (HPV, HIV, EBV, Zika, and Chikungunya).
According to UNAIDS, the number of adults and children living with HIV in the Latin American region reached 2,100,000 in 2020 from 2,000,000 in 2018. The number of annual new infections has continued to rise in the region. Furthermore, the outbreak of COVID-19 disease had affected a large population in the region and spread continuously throughout the region. Apart from infectious diseases, non-communicable diseases (NCDs), including cancer, are the leading cause of preventable and premature deaths in Latin America. In 2020, cancer cases in Latin America were 1.47 million, which is estimated to reach 1.93 million by 2030 and 2.44 million by 2040 (Source: GLOBCAN)
This increase in the number of diseases in the region is fueling the growth of the Latin America IVD market.
Based on Product & Solution, the Kits & Reagents Segment is Expected to Register the Highest CAGR during the Forecast Period
Factors such as the increasing prevalence of infectious diseases in the region and the increasing use of kits & reagents to identify the organism causing various diseases in less time and cost contribute to the high growth of this market. Furthermore, the outbreak of COVID-19 disease accelerated the growth of this segment due to the national emergency and the requirement of various kits & assays for early disease diagnosis.
Based on Technology, the Molecular Diagnostics Segment is Expected to Account for the Largest Share of Latin America In Vitro Diagnostics Market
The high prevalence of infectious diseases, advantages of molecular diagnostics techniques such as high sensitivity and accuracy, and rising penetration of advanced molecular techniques are factors driving the growth of this segment.
Based on Diagnostic Approach, the Lab Testing Segment is Estimated to Account for the Largest Share in 2023
The lab testing approach for IVD has higher accuracy and reliability than point-of-care testing. The laboratory equipment and analyzers have high sensitivity and specificity. The lab testing segment is expected to account for the largest share of the Latin America IVD market due to its higher accuracy and reliability, lower costs, availability of several IVD tests, and quality assurance program.
Based on End User, the Hospitals & Clinics are Estimated to Account for the Largest Share in 2023
Hospitals & Clinics can increase the economies of scale and maximize the usage of equipment and staff while not reducing the level of service. Due to this reason, hospital laboratories are one of the major end users for IVD in Latin America.
Brazil is Expected to Dominate the Latin American IVD Market
Factors such as well-established healthcare infrastructure, growing healthcare expenditure, rising demand for genetic testing, increasing awareness about the use of IVD products, and growing awareness for cancer screening are responsible for the growth of this segment.
Key Players
The report includes a competitive landscape based on an extensive assessment of the product portfolio offerings, geographic presences, and key strategic developments in leading industry market players over the past three to four years. The key players operating in the IVD market in Latin America are F. Hoffmann-La Roche Ltd. (Switzerland), Siemens Healthineers AG (Germany), Abbott Laboratories (U.S.), Danaher Corporation (U.S.), Becton, Dickinson and Company (U.S.), bioMérieux SA (France), Qiagen N.V. (Netherlands), Thermo Fisher Scientific Inc. (U.S.), Wama Diagnóstica (Brazil), and Wiener Laboratorios SAIC (Argentina)
Scope of the Report:
Latin America In Vitro Diagnostics Market Assessment, by Product & Solution
Latin America In Vitro Diagnostics Market Assessment, by Technology
Latin America In Vitro Diagnostics Market Assessment, by Application
Latin America In Vitro Diagnostics Market Assessment, by Diagnostic Approach
Latin America In Vitro Diagnostics Market Assessment, by End User
Latin America In Vitro Diagnostics Market Assessment, by Country
Key questions answered in the report-
The Latin America in vitro diagnostics market covers the market size & forecasts of reagents, kits, assays, instruments/analyzers, and software/services used to diagnose diseases. Latin America in vitro diagnostics market studied in this report involves the value analysis of various segments and sub-segments of in vitro diagnostics at the country level.
The Latin America in vitro diagnostics market is projected to reach $6.45 billion by 2030, at a CAGR of 5.5% during the forecast period.
Based on products & solutions, the kits & reagents segment is estimated to account for the largest share of the Latin America in vitro diagnostics market in 2023. Factors such as the increasing prevalence of infectious diseases in the region and the increasing use of kits & reagents to identify the organism causing various diseases in less time and cost contribute to the high growth of this market.
Based on application, the cardiology segment is expected to grow at the fastest CAGR during the forecast period due to the increasing prevalence of various cardiovascular diseases and new innovative product launches for the cardiology segment.
Based on technology, the molecular diagnostics segment held the largest market share due to its advantages, such as high sensitivity and accuracy, high adoption, and high prevalence of infectious diseases in the region.
The growth of the in vitro diagnostics market in the region is attributed to the growing prevalence of communicable and non-communicable diseases, growing medical tourism, adoption of point of care testing, and growing awareness regarding early disease diagnosis. On the other hand, the inclination towards personalized healthcare and advances in genomics and proteomics offer opportunities for the IVD market in Latin America.
The key players operating in the IVD market in Latin America are F. Hoffmann-La Roche Ltd. (Switzerland), Siemens Healthineers AG (Germany), Abbott Laboratories (U.S.), Danaher Corporation (U.S.), Becton, Dickinson and Company (U.S.), bioMérieux SA (France), Qiagen N.V. (Netherlands), Thermo Fisher Scientific Inc. (U.S.), Wama Diagnóstica (Brazil), and Wiener Laboratorios SAIC (Argentina).
Countries such as Brazil, Argentina, Peru, Colombia, and Chile are projected to offer significant growth opportunities to the vendors in this market due to their well-established healthcare infrastructure and growing healthcare expenditure.
























Published Date: Apr-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Jan-2025
Published Date: Feb-2024
Please enter your corporate email id here to view sample report.
Subscribe to get the latest industry updates